It is a process utilized to change certain characteristics of metals and alloys in order to make them more suitable for a particular kind application Heat Treatment can greatly influence mechanical properties such as strength, hardness, ductility, toughness, and wear resistance of the alloys.
Heat Treatment of Carbon Steels and Carbon Alloy Steels:

Heat Treatment on both type of the steel is done for improving mechanical properties such as tensile and yield strength. This is accomplished by altering the molecular structure of steel in order to produce more durable microstructure. The structure of steel is composed of two variables:
| Grain Structure: The arrangement of atoms in a metal. | |
| Grain Size: The size of the individual crystals of metal. Large grain size is generally associated with low strength, hardness, and ductility. |
During the alloy process elements such as carbon are introduced to the metal. These added elements interrupt the flow of the individual grains, increasing strength. Thus, control of the metal crystal structure is a key element in successful heat treating.
A Metal can also exist in various phases: Ferrite, austenite and cementite. To better understand these phases, look at the Iron-Carbon Phase Diagram. The Y-axis (vertical) is a measurement of temperature while the X-Axis (Horizontal) is a measurement of the carbon content of the steel. The far left hand side of the X-axis represents the Ferrite phase of steel (low carbon content) while the far right hand. Side represents the cementite phase of steel (high carbon content), which is also known as iron carbide. The curved horizontal line that occurs just above 1333 ºF represents the austenite phase of steel.
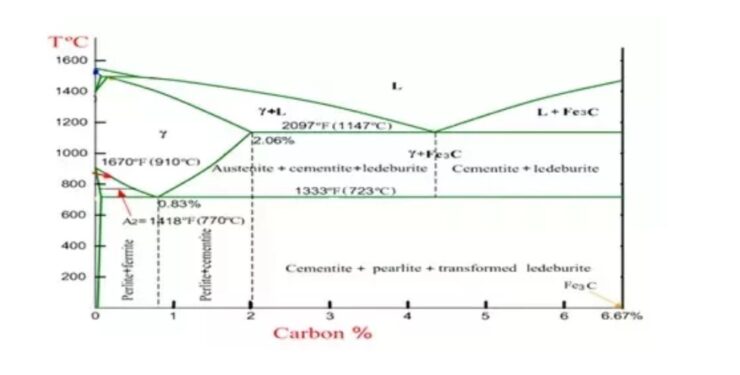
The following phases are involved in the transformation, occurring with iron-carbon alloys:
- L –L represents a liquid solution of carbon in iron.
- δ-ferrite – δ-ferrite is a solid solution of carbon in iron. Maximum concentration of carbon in δ-ferrite is 0.09% at 2719 ºF (1493ºC) – temperature of the peritectic transformation. The crystal structure of δ-ferrite is BCC (cubic body centered).
- Austenite –Austenite is an interstitial solid solution of carbon in γ-iron. Austenite has FCC (cubic face centered) crystal structure, permitting high solubility of carbon – up to 2.06% at 2097 ºF (1147 ºC). Austenite does not exist below 1333 ºF (733ºC) and maximum carbon concentration at this temperature is 0.83%.
- α-ferrite – α-ferrite is a solid solution of carbon in α-iron. α-ferrite has BCC crystal structure and low solubility of carbon – up to 0.25% at 1333 ºF (733ºC). It exists at room temperature.
- Cementite – Cementite is an iron carbide, intermetallic compound, having fixed composition Fe3C. Cementite is a hard and brittle substance, influencing on the properties of steels and cast irons.
When ferrite (low carbon steel) is at room temperature, it has a body-centered-cubic structure, which can only absorb a low amount of carbon. Because Ferrite can only absorb a very low amount of carbon at room temperature, the un-absorbed carbon separates out of the body-centered-cubic structure to form carbides which join together to create small packets of an extremely hard crystal structure within the ferrite called cementic. However, when ferrite is heated to a temperature above the transformation line( austenite line) the body-centered-cubic structure changes to a face-centered-cubic structure, thus allowing for absorption of the carbon into the crystal structure.
Once the steel enters the austenitic phase all of the cementite dissolves into austenite. If the steel is allowed to cool slowly, the carbon will separate out of the ferrite as a cubic-structure reverts from face-centered back to body-centered. The islands of cementite will reform within the ferrite, and the steel will have the same properties that it did before it was heated. However, when the steel is rapidly cooles, or quenched, in a quenching medium (such as oil, water or cold air) the carbon does not have time to exit the cubic structure of the ferrite and it becomes trapped within it. This leads to the information of martensitic; microstructure that produces the most sought after mechanical properties in steel fasteners.
During quenching, it is impossible to cool the specimen at a uniform rate throughout. The surface will always cool more rapidly than the interior of the specimen. Therefore, the austenite will transform over a range of temperatures, yielding a possible variation of microstructure and properties depending on the position within the material.
The successful heat treatment of steels to produce a predominantly martensitic micro structure throughout the cross section depends mainly on three factors:
- The composition of the alloy.
- The type and character of the quenching medium.
- The size and shape of the specimen.
Read More: Heat Treatment Terminology
Hardenability :
It is the ability of steel to transform into martensite with a particular quenching treatment. This is directly affected by the alloy composition of the steel. For every different steel alloy there is a specific relationship between its mectanical properties and its cooling rate. It is not “hardness” which is a resistance to indentation; rather, hardness measurements are utilized to determine the extent of a martensitic transformation in the interior of the material.
Tempering :
It involves heating the steel to a specific temperature below that of ausenite and allowing it to cool slowly. This cause the crystal structure to relax, thereby increasing the ductility and decreasing the hardness to specified levels. The specific tempering temperature will vary based on the desired result for the steel.
The following example will demonstrate the effectiveness of the tempering:
ASTM A193 Grade B7 , SAE J429 Grade and ASTM A574 Socket Head cap Screws are all made from alloy steels. In fact some alloy steel grades can be used to manufacture any of the three final products. Such as 4140 and 4142 alloy steel. The final mechanical properties apper in the table.
| Fasteners Produced From AISI 4140& 4142 Steel | |||
| Fastener | ASTM A193 B7 | SAE J429 Gr. 8 | ASTM A574 SHCS |
| Tempering Temp. |
1150◦F | 800◦F | 650◦F |
| Tensile strength | 125,000 PSI min (2 1\2in and under) | 150,000 PSI min | 180,000 PSI min ( through ½ in ) 170,000 PSI min ( above ½ in) |
| Yield Strength |
105,000 PSI min (2 1/2in and under) |
130,000 PSI min | 153,000 PSI min |
| Proof Strength |
N/A | 120,000 PSI | 140,000 PSI min ( through ½ in ) 135,000 PSI min ( above ½ in ) |
| Hardness | HRC 35 max. | HRC 33-39 | HRC 39-45 ( through ½ in ) HRC 37-45 ( above ½ in ) |
The initial heat treating process is relatively the same for the entire three products. The parts are heat treated until fully austenitized and then are quenched and tempered in the oil. This tempering temperature dictates the final product. A lower tempering temperature will produce a harder and higher tensile strength part for these alloys steels. However, the lower tempering temperatures will also mean lower ductility, impact strength, and possibly lower fatigue life.
Annealing

It is the heat treating process used to soften previously cold-worked metal by allowing it to re-crystallize.The term annealing refers to a heat treatment in which a material is exposed to an elevated tempertature for an extended period and then slowly cooled. Ordinarily, annealing is carried out to, (1) Relieve stress (often introduced when cold-working the part.) ;(2) Increase softness, ductility and toughness; and (3) Produce a desired microstructure.
Any annealing process consists of the three stages:
- Heating to the desired temperature
- Holding or “soaking” at that temperature
- Slowly cooling, usually to room temperature
Time is the important parameters in these procedures. It is used to negate the effects of cold work that is to be soften and increase the ductility of a previous strain-hardened metal.
Stress relieving
It is a process that is utilized when internal residual stress develop in metal pieces in response to such thing as cold working. Failure to remove these internal stress may result in distottion and warping. A stress relief anneaing heat treatment removes these stress heating the piece to a recommended temperature, held there long enough to attain a uniform temperature, and finally cooled to a room temperature in air.
Normalizing
It is an annealing heat treatment used to refine the grains and produce a more uniform and desirable size distribution. Medium and high carbon steels having microstructure containing pearlite may still be too hard to conveniently machine or plastically deform. These steels (and in fact,any steel) may be annealted to develop the spheroidite structure . Spheroidized steels have a maximum softness and ducility and are easlly machined or deformed.

